Bioanalytical Testing Services Market Size to Hit US$ 6.9 Billion by 2032 | Grow CAGR by 9%
Bioanalytical Testing Services Market Size
Summary:
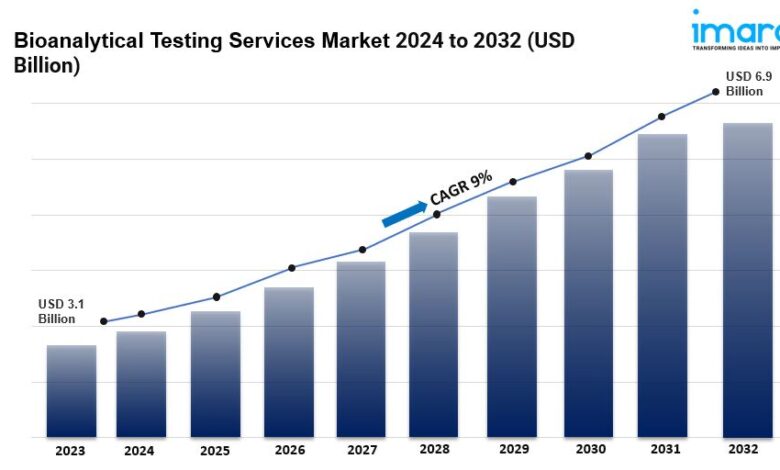
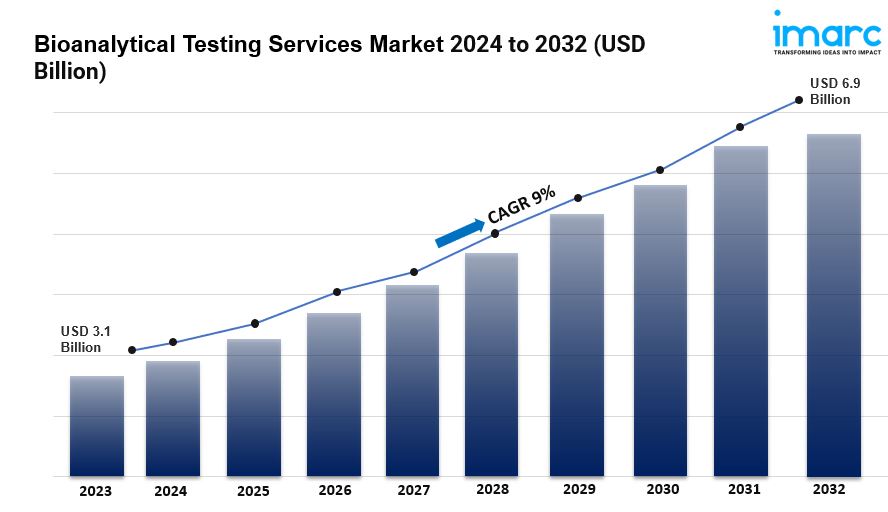
- The global bioanalytical testing services market size reached US$ 3.1 Billion in 2023.
- The market is expected to reach US$ 6.9 Billion by 2032, exhibiting a growth rate (CAGR) of 9% during 2024-2032.
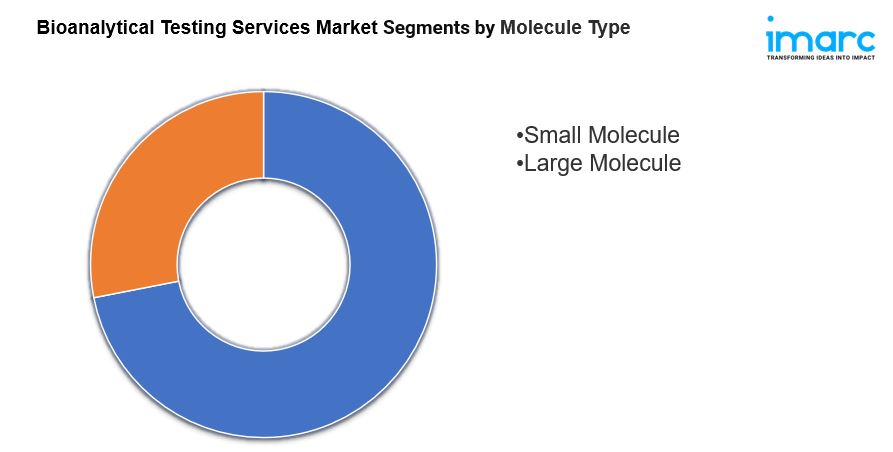
- Based on the molecule type, the market has been bifurcated into small molecule and large molecule (LC-MS studies, immunoassays, and others).
- On the basis of test type, the market has been classified into ADME (in-vivo, and in-vitro), PK, PD, bioavailability, bioequivalence, and others.
- Based on the workflow, the market has been categorized into sample preparation (protein precipitation, liquid-liquid extraction, and solid phase extraction), sample analysis (hyphenated technique, chromatographic technique, electrophoresis, ligand binding assay, mass spectrometry, and nuclear magnetic resonance), and others.
- On a regional basis, the market has been segmented into North America (the United States and Canada), Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others), Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others), Latin America (Brazil, Mexico, and others), and Middle East and Africa.
- The increasing regulatory scrutiny and stringent guidelines for drug safety and efficacy, which are pushing pharmaceutical companies to ensure compliance through comprehensive testing are driving the growth of the bioanalytical testing services market.
- Technological advancements, such as automation and high-throughput screening, are enhancing the speed and accuracy of bioanalytical testing and making it more efficient, thus creating a positive outlook for market expansion.
Industry Trends and Drivers:
Increasing drug development activities: One of the primary drivers of the bioanalytical testing services market is the surge in drug development activities. Pharmaceutical and biotechnology companies are continuously working to develop new and more effective drugs to meet the rising demand for treatment options across various disease areas. This surge is particularly evident in the development of new therapies, such as small molecules, biologics, and biosimilars. The growing prevalence of chronic diseases like cancer, diabetes, cardiovascular disorders, and autoimmune diseases is further intensifying the need for new therapeutic solutions. As more compounds enter clinical trials, there is a heightened demand for bioanalytical testing to assess the pharmacokinetics (PK), pharmacodynamics (PD), safety, and efficacy of these drugs. These tests are essential for determining the proper dosing, understanding drug metabolism, and evaluating potential toxicity, which is critical for drug approval processes by regulatory authorities such as the FDA and EMA.
Growing complexity of biologics: Biologics, which include monoclonal antibodies, vaccines, cell and gene therapies, and recombinant proteins, have revolutionized the treatment landscape for many complex diseases. However, the development of biologics is significantly more complicated than traditional small-molecule drugs due to their large molecular size, complex structure, and intricate manufacturing processes. This complexity necessitates highly specialized bioanalytical testing methods to ensure product quality, safety, and efficacy. Unlike small molecules, which are easier to characterize chemically, biologics require advanced analytical techniques to assess their purity, potency, and stability. Bioanalytical testing services offer the sophisticated instrumentation and expertise needed to analyze these complex molecules at every stage of the development process. This growing reliance on biologics has fueled the demand for advanced bioanalytical services, as pharmaceutical companies often lack the in-house capability to handle such intricate analysis.
Rise in outsourcing of bioanalytical services: The outsourcing of bioanalytical testing services has become increasingly common among pharmaceutical and biotechnology companies. Drug development is a highly resource-intensive process that requires significant investment in terms of time, expertise, and equipment. By outsourcing bioanalytical testing, companies can focus on core activities such as drug discovery and clinical development while relying on specialized contract research organizations (CROs) to handle the complex and time-consuming bioanalytical tasks. This trend is particularly beneficial for small and mid-sized companies that may not have the internal infrastructure or resources to perform these tests in-house. Outsourcing offers access to cutting-edge technology, expert personnel, and faster turnaround times, which are critical for improving drug development timelines and maintaining competitive advantage in the market.
Request for a sample copy of this report: https://www.imarcgroup.com/bioanalytical-testing-services-market/requestsample
Bioanalytical Testing Services Market Report Segmentation:
Breakup By Molecule Type:
- Small Molecule
- Large Molecule
On the basis of the molecule type, the market has been bifurcated into small molecule and large molecule (LC-MS studies, immunoassays, and others).
Breakup By Test Type:
- ADME
- PK
- PD
- Bioavailability
- Bioequivalence
- Others
Based on the test type, the market has been classified into ADME (in-vivo, and in-vitro), PK, PD, bioavailability, bioequivalence, and others.
Breakup By Workflow:
- Sample Preparation
- Sample Analysis
- Others
On the basis of the workflow, the market has been categorized into sample preparation (protein precipitation, liquid-liquid extraction, and solid phase extraction), sample analysis (hyphenated technique, chromatographic technique, electrophoresis, ligand binding assay, mass spectrometry, and nuclear magnetic resonance), and others.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position owing to a large market for blinds and shades driven by high consumer spending on home decor and renovation.
Top Bioanalytical Testing Services Market Leaders:
The keyword market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
- Almac Group
- Charles River Laboratories International Inc.
- Frontage Laboratories Inc.
- ICON plc
- Intertek Group plc
- Laboratory Corporation of America Holdings
- Pace Analytical Services LLC
- PPD Inc.
- PRA Health Sciences
- SGS SA
- Syneos Health
- Toxikon Corporation
Browse full report with TOC & List of Figures: https://www.imarcgroup.com/bioanalytical-testing-services-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.